Abstract
Background: Over a third of pts with 1L DLBCL do not respond to, or relapse after, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP; [Sarkozy and Sehn. Ann Lymphoma 2019]). Despite recent advances, pts with R/R NHL have limited curative options. Glofitamab (Glofit) is a novel, T-cell-engaging bispecific antibody with a 2:1 molecular configuration that allows bivalent binding to CD20 on B cells and monovalent binding to CD3 on T cells. Unlike other CD20xCD3 bispecific antibodies, this format uniquely enables combination with anti-CD20 antibodies, including rituximab. Glofit monotherapy induces high response rates in R/R B-cell NHL (Hutchings et al. J Clin Oncol 2021). We present results of the ongoing NP40126 study (NCT03467373), designed to assess the feasibility and safety of Glofit + R-CHOP in R/R NHL (dose-escalation phase) and 1L DLBCL (safety run-in phase).
Methods: R/R NHL dose-escalation: Pts (Eastern Cooperative Oncology Group performance status [ECOG PS] 0-2) received increasing Glofit doses in separate cohorts (70µg, 1800µg, 10mg and 30mg) plus standard R-CHOP for 6-8 cycles (each 21-day). To mitigate CRS risk, R- or obinutuzumab (G)-CHOP was given in Cycle (C)1, with the aim of tumor debulking. Glofit was given from C2 onwards. For 70µg and 1800µg cohorts, fixed-dose Glofit was given on C2 Day (D)8 and onwards. For 10mg and 30mg cohorts, step-up dosing was used to further mitigate CRS risk (2.5mg C2D8, 10mg C2D15, target dose C3D8 and onwards). Optional Glofit maintenance was permitted (every 2 months for <2 years; dose-escalation phase only).
1L DLBCL safety run-in: Pts (ECOG PS 0-3) received Glofit 30mg plus standard R-CHOP for 6-8 cycles (each 21-day). Pts received R-CHOP in C1; Glofit step-up dosing began in C2 (2.5mg C2D8, 10mg C2D15, 30mg C3D8 and onwards).
Response rates were assessed by PET-CT (Lugano criteria; [Cheson et al. J Clin Oncol 2014]). CRS events were graded by ASTCT criteria [Lee et al. Biol Blood Marrow Transplant 2019].
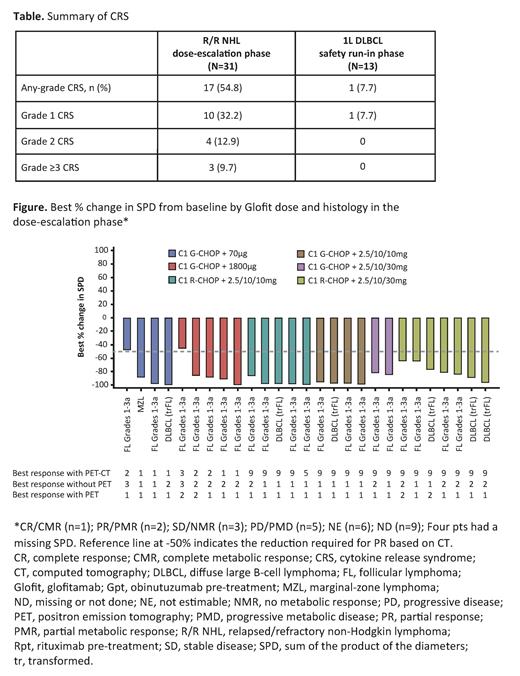
Results:
R/R NHL dose-escalation: At data cut-off (June 10, 2021), 31 pts (23 follicular lymphoma [FL]; 6 transformed FL; 1 marginal-zone lymphoma; 1 mantle-cell lymphoma) had received Glofit with R/G-CHOP. Median age was 62 years, median prior lines of therapy was 2 (range: 1-5). In efficacy-evaluable pts (n=31), after a median 9.0 months' (range: 0-29) follow-up, the overall response rate (ORR) was 90% (n=28) and complete response rate (CRR) was 77% (n=24). Median duration of response was not reached. The Figure shows change in tumor size. Grade (Gr) ≥3 adverse events (AEs) occurred in 28 (90%) pts, serious AEs in 21 (68%) pts and CRS in 17 (55%) pts (mostly low grade; majority after the first 2.5mg Glofit dose; Table). One (3%) pt had a Gr 5 AE (COVID-19 pneumonia not related to study treatment). AEs led to Glofit dose modification/interruption in 2 (6%) pts and Glofit withdrawal in 1 (3%) pt. Neurologic AEs (NAEs) occurred in 20 (65%) pts: Gr 1-2 (16 pts, 52%); Gr 3 (4 pts, 13%). Immune effector cell-associated neurotoxicity syndrome (ICANS)-like AEs were uncommon; a serious AE was reported in 1 pt only (Gr 3 epilepsy during the maintenance phase; resolved in 3 days). Neutropenia occurred in 24 (77%) pts. Median dose intensity was 100% for all R-CHOP components.
1L DLBCL safety run-in: At data cut-off, 13 pts were enrolled (safety population); of these, 4 pts received Glofit 30mg with R-CHOP and were efficacy-evaluable. Median age was 68 years, all pts had Ann Arbor Stage 3/4 disease. At interim assessment (C3), CRR was 100% (4/4). Of 13 pts, 1 (8%) had a CRS event (Gr 1 with fever only) after the first 2.5mg Glofit dose; no other CRS events observed. Gr ≥3 AEs occurred in 8 (62%) pts and Gr ≥3 AEs related to Glofit in 1 (8%) pt only. One (8%) pt had a serious AE and 1 (8%) pt had a Gr 5 AE (infusion-related reaction related to rituximab on C1D1). No AEs led to Glofit or R-CHOP dose interruptions. NAEs occurred in 3 (23%) pts (all Gr 1-2; none were ICANS-like). Neutropenia occurred in 6 (46%) pts. Median dose intensity was 100% for all R-CHOP components.
Conclusions: Initial data show that Glofit + R-CHOP has tolerable safety in R/R NHL and 1L DLBCL. R-CHOP dose intensity was maintained in all pts. The very low CRS rate and no neurotoxicity in 1L DLBCL may render Glofit particularly suitable for the outpatient setting without the need for hospitalization. Updated data, including end-of-treatment responses from the 1L DLBCL safety run-in phase, will be presented.
Ghosh: Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau; Karyopharma: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria; Adaptive Biotech: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Epizyme: Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Genentech: Research Funding. Townsend: Celgene (Bristol-Myers Squibb): Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria. Dickinson: Amgen: Honoraria; Celgene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Research Funding; Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: travel, accommodation, expenses, Research Funding, Speakers Bureau. Topp: Celgene: Consultancy, Research Funding; Janssen: Consultancy; Universitatklinikum Wurzburg: Current Employment; Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy; Roche: Consultancy, Research Funding; Gilead: Research Funding; Regeneron: Consultancy, Research Funding; Macrogeniecs: Research Funding; Amgen: Consultancy, Research Funding. Santoro: Sandoz: Speakers Bureau; Eli-Lilly: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; AbbVie: Speakers Bureau; Roche: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Sanofi: Consultancy; Arqule: Consultancy, Speakers Bureau; Novartis: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Crump: Novartis: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Membership on an entity's Board of Directors or advisory committees; Epizyme: Research Funding; Roche: Research Funding. Morschhauser: Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy; AstraZenenca: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria. Mehta: Kite/Gilead; Roche-Genetech; Celgene/BMS;Oncotartis; Innate Pharmaceuticals; Seattle Genetics;Incyte; Takeda; Fortyseven Inc/Gilead; TG Therapeutics;Merck; Juno Pharmaceuticals/BMS: Research Funding; Seattle Genetics; Incyte; TG Therapeutics: Consultancy; Seattle Genetics; Incyte; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Panchal: F. Hoffmann-La Roche Ltd: Current Employment. Wu: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Barrett: Roche Products Ltd: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Humphrey: Roche: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Qayum: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Hutchings: Novartis: Research Funding; Janssen: Honoraria, Research Funding; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Celgene: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria, Research Funding.
Glofitamab is a full-length, humanized immunoglobulin G1 bispecific antibody with a 2:1 molecular format that facilitates bivalent binding to CD20 on B-cells, and monovalent binding to CD3 on T-cells. Glofitamab redirects T cells to engage and eliminate malignant B cells. Glofitamab is an investigational agent. Rituximab (Rituxan) is a CD20-directed cytolytic antibody indicated for the treatment of adult pts with: relapsed or refractory, low grade or follicular, CD20-positive, B-cell NHL as a single agent; previously untreated follicular, CD20-positive, B-cell NHL in combination with first-line chemotherapy (chemo) and, in pts achieving a CR or PR to a rituximab product in combination with chemo, as single-agent maintenance therapy; non-progressing (including stable disease), low-grade, CD20 positive, B-cell NHL as a single agent after first-line CVP chemo; previously untreated diffuse large B-cell, CD20-positive, NHL in combination with CHOP or other anthracycline-based chemo regimens; previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide.